This litigation has concluded and we are proud to report that our clients' claims have been successfully resolved. The amounts of all individual client recoveries are confidential, both by settlement agreement and by attorney-client privilege. MLG is no longer accepting cases involving this product.
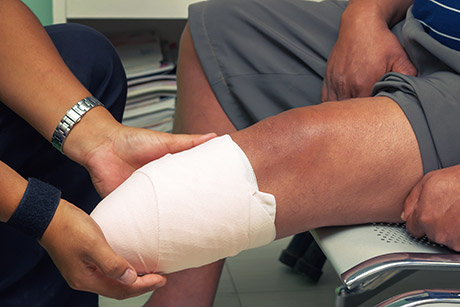 In 2017 the FDA issued a Drug Safety Communication confirming that patients taking Invokana®, Invokamet® and Invokamet® XR are at an increased risk of leg and foot amputations and that the manufacturers (Johnson & Johnson and its subsidiary Janssen Pharmaceuticals) must include a “Black Box Warning” on the drugs’ labeling.
In 2017 the FDA issued a Drug Safety Communication confirming that patients taking Invokana®, Invokamet® and Invokamet® XR are at an increased risk of leg and foot amputations and that the manufacturers (Johnson & Johnson and its subsidiary Janssen Pharmaceuticals) must include a “Black Box Warning” on the drugs’ labeling.
The Drug Safety Communication was issued after the FDA reviewed data from two different clinical trials – the CANVAS and CANVAS-R studies. These data demonstrated that diabetes patients treated with Invokana®, Invokamet® and Invokamet® XR were twice as likely to require leg and foot amputations than those diabetes patients who were taking placebo (a non-drug).
| Invokana® | Placebo | Difference | Increased Risk | |
|---|---|---|---|---|
| Trial #1 | 5.9 | 2.8 | 3.1 | 2.11 |
| Trial #2 | 7.5 | 4.2 | 3.3 | 1.79 |
In the two studies, amputations of the toe and middle foot were the most common (~ 70%) but both leg and below/above the knee amputations also occurred (30%). Some patients had more than one amputation and some had amputations involving both limbs.
After reviewing data from the two clinical trials, the FDA required the addition of the following Boxed Warning to the labeling for Invokana®, Invokamet® and Invokamet® XR:
On August 29, 2018 the FDA issued an urgent Safety Announcement to health care professionals and their patients. The Alert warned about the association between Invokana®/Invokamet®/Invokamet XR®, Jardiance®, Farxiga® and other SGLT2 diabetes medications and Fournier’s Gangrene, a life-threatening genital infection that can affect both men and women. Invokana®, Farxiga® and Jardiance® are included in the class of drugs known as sodium-glucose cotransporter-2 (SGLT2) inhibitors which are prescribed for the treatment of Type 2 Diabetes.
SGLT2 inhibitors work by promoting the removal of glucose through the kidneys. This means that a patient’s urine contains a high volume of sugar, which creates a bodily environment very conducive for the growth of bacteria. Though SGLT2 inhibitors such as Invokana®, Farxiga® and Jardiance® have been associated with an abnormally higher incidence of urinary tract infections, the association with Fournier’s Gangrene appears to be a recent revelation to the FDA.
Necrotizing fasciitis of the genitals and genital area, commonly known as Fournier’s Gangrene, is an uncommon bacterial infection that can develop in the scrotum, penis, labia, perineum, essentially any area from the genitals to the rectum. “Necrotizing” means that unchecked, the infection can destroy flesh so treatment of advanced cases usually involves surgery to stop its spread. If left untreated, Fournier’s Gangrene can lead to amputations and death.
According to the Centers for Disease Control (the “CDC”), the first onset of symptoms can be “confusing” since a red or swollen area of the skin is first to appear. However, swelling and fever can develop quickly. The FDA warns that:
“Patients should seek medical attention immediately if you experience any symptoms of tenderness, redness, or swelling of the genitals or the area from the genitals back to the rectum, and have a fever above 100.4 F or a general feeling of being unwell. These symptoms can worsen quickly, so it is important to seek treatment right away.”
In October, 2018, all of the SCLT2 Inhibitors - Invokana®/Invokamet®/Invokamet® XR, Jardiance®/Glyxambi®/Synjardy®/Synjardy® XR, Farxiga®/Xigduo® XR/Qtern®, Stelgato®/Steglatro®/Segluromet®/Steglujan® added the following warning to their Prescribing Information:
Necrotizing Fasciitis of the Perineum (Fournier’s Gangrene) Reports of necrotizing fasciitis of the perineum (Fournier’s gangrene), a rare but serious and life-threatening necrotizing infection requiring urgent surgical intervention, have been identified in postmarketing surveillance in patients with diabetes mellitus receiving SGLT2 inhibitors. Cases have been reported in both females and males. Serious outcomes have included hospitalization, multiple surgeries, and death. Patients treated with SGLT2 inhibitors presenting with pain or tenderness, erythema, or swelling in the genital or perineal area, along with fever or malaise, should be assessed for necrotizing fasciitis. If suspected, start treatment immediately with broad-spectrum antibiotics and, if necessary, surgical debridement. Discontinue SGLT2 inhibitor use, closely monitor blood glucose levels, and provide appropriate alternative therapy for glycemic control.
This litigation has concluded and we are proud to report that our clients' claims have been successfully resolved. The amounts of all individual client recoveries are confidential, both by settlement agreement and by attorney-client privilege. MLG is no longer accepting cases involving this product.